Safety & dosing considerations for ROCKLATAN®
(netarsudil and latanoprost ophthalmic solution) 0.02%/0.005%
Setting patient expectations about hyperemia
What to know about hyperemia
- ROCK inhibitors may cause vasodilation on the ocular surface1
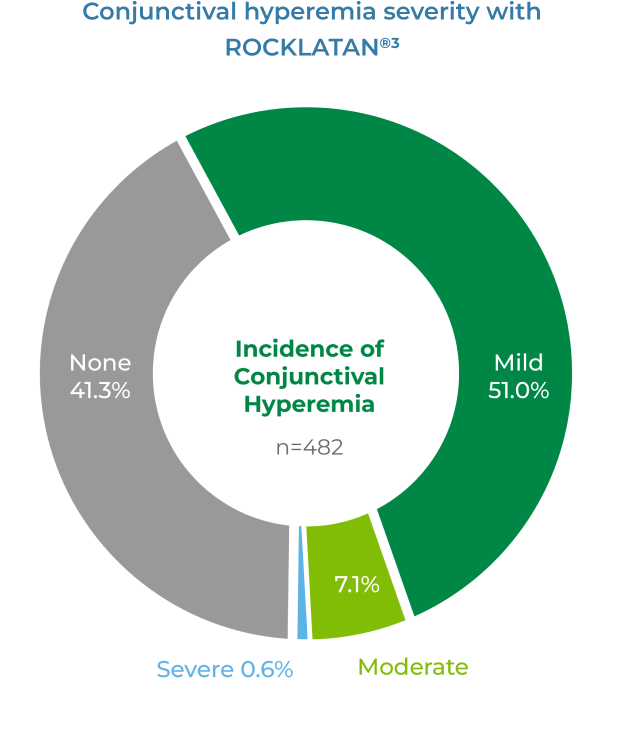
- 59% of patients experienced hyperemia in clinical studies; however, only 5% of patients discontinued for this reason2
What patients should know about hyperemia
- ROCKLATAN® causes blood vessels in the eye to dilate, which may result in visible redness1
- Redness alone does not necessarily indicate an allergic reaction. Instruct patients to inform you if they develop inflammation or irritation of the eye or eyelids
Most occurrences of hyperemia were rated by investigators as mild to moderate3
See Important Safety Information about ROCKLATAN® on this page.
Help your patients get the most out of their ROCKLATAN® treatment
Help your patients get the most out of their ROCKLATAN® treatment with the MyAlcon Together Patient Support program
ROCKLATAN® should be taken once daily in the evening2
- The recommended dose for ROCKLATAN® is one drop in the affected eye(s) once daily in the evening2
- If you miss a dose of ROCKLATAN®, you should continue with your next dose the following evening2
- If more than 1 eye drop is being used, the drugs should be administered at least 5 minutes apart2
- Contact lenses should be removed prior to the administration of ROCKLATAN® and may be reinserted 15 minutes after administration2
- Avoid allowing the tip of the bottle to contact the eye, surrounding structures, fingers, or any other surface in order to minimize contamination of the solution. Serious damage to the eye and subsequent loss of vision may result from using contaminated solution
- Protect ROCKLATAN® from light. Until opened, store ROCKLATAN® at 36 °F to 46 °F. After opening, the product may be kept at 36 °F to 77 °F for up to 6 weeks. If after opening the product is kept refrigerated at 36 °F to 46 °F, then the product can be used until the expiration date stamped on the bottle
ROCK=Rho kinase.
REFERENCES
1. Brubaker JW, Teymoorian S, Lewis RA, et al. One year of netarsudil and latanoprost fixed-dose combination for elevated intraocular pressure: phase 3, randomized MERCURY-1 study. Ophthalmol Glaucoma. 2020;3(5):327-338.
2. Rocklatan® (netarsudil and latanoprost ophthalmic solution) 0.02%/0.005% Prescribing Information. Aerie Pharmaceuticals, Inc., Irvine, CA. 2020.
3. Alcon data on file, 2017.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
- Epithelial corneal edema, described as honeycomb or bullous, has been reported in some patients with pre-existing corneal stromal edema or following ocular procedures that could affect corneal endothelial function. Epithelial corneal edema typically resolves upon discontinuation of ROCKLATAN®. Advise patients to notify their physician if they experience eye pain or decreased vision while using ROCKLATAN®.
INDICATIONS AND USAGE
ROCKLATAN® (netarsudil and latanoprost ophthalmic solution) 0.02%/0.005%, is a fixed dose combination of a Rho kinase inhibitor and a prostaglandin F2α analogue indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
- Increased pigmentation of the iris, periorbital tissue (eyelid), and eyelashes can occur. Iris pigmentation likely to be permanent.
- Gradual change to eyelashes may include increased length, thickness, number, and misdirected growth of lashes. Usually reversible upon discontinuation of treatment.
- Use with caution in patients with a history of intraocular inflammation (iritis/uveitis). Should generally not be used in patients with active intraocular inflammation.
- Macular edema, including cystoid macular edema, has been reported with latanoprost. Use with caution in aphakic patients, pseudophakic patients with a torn posterior lens capsule, or patients with known risk factors for macular edema.
- Use with caution in patients with a history of herpetic keratitis. Avoid use in cases of active herpes simplex keratitis.
- There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface.
- Contact lenses should be removed prior to the administration of ROCKLATAN® and may be reinserted 15 minutes after administration.
INDICATIONS AND USAGE
ROCKLATAN® (netarsudil and latanoprost ophthalmic solution) 0.02%/0.005% is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
Adverse Reactions
The most common ocular adverse reaction observed in controlled clinical studies with ROCKLATAN® was conjunctival hyperemia which was reported in 59% of patients. Five percent of patients discontinued therapy due to conjunctival hyperemia. Other common ocular adverse reactions reported were: instillation site pain (20%), corneal verticillata (15%), and conjunctival hemorrhage (11%). Eye pruritus, visual acuity reduced, increased lacrimation, instillation site discomfort, and blurred vision were reported in 5-8% of patients.
Please click here for the ROCKLATAN® Full Prescribing Information.
