Discover the complementary MOAs of ROCKLATAN®
(netarsudil and latanoprost ophthalmic solution) 0.02%/0.005%
The problem of reduced outflow
In open-angle glaucoma patients, IOP becomes elevated when the trabecular meshwork becomes fibrotic and aqueous humor outflow is reduced.1
- The trabecular meshwork is integral to the regulation of aqueous humor outflow and IOP2
- Cellular damage due to glaucoma (e.g., oxidative stress) may result in stiffness of the trabecular meshwork3
- This may impede the outflow of aqueous humor and result in persistently high IOP3
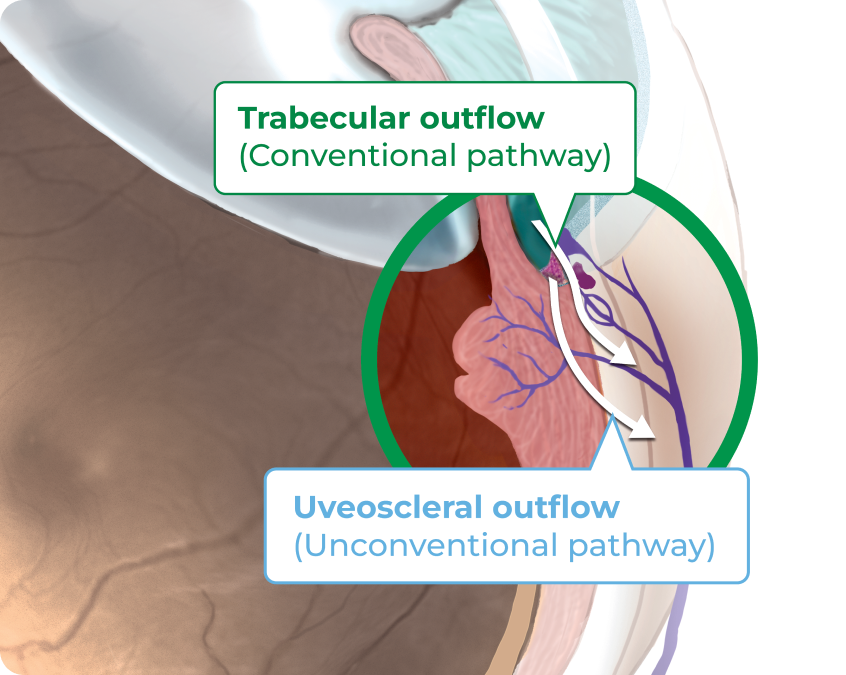
ROCKLATAN® contains latanoprost and netarsudil, which are believed to reduce IOP by increasing aqueous humor outflow through the conventional and unconventional pathways4
The exact mechanism is unknown.
IOP=Intraocular pressure;
MOAs=Mechanisms of action.
REFERENCES
1. Winkler NS, Fautsch MP. Effects of prostaglandin analogues on aqueous humor outflow pathways. J Ocul Pharmacol Ther. 2014;30(2-3):102-109.
2. Saccà SC, Pulliero A, Izzotti A. The dysfunction of the trabecular meshwork during glaucoma course. J Cell Physiol. 2014;230:510-525.
3. Ren R, Li G, Duong Le T, Kopczynski C, Stamer WD, Gong H. Netarsudil increases outflow facility in human eyes through multiple mechanisms. Invest Ophthalmol Vis Sci. 2016;57(14):6197-6209.
4. Brubaker JW, Teymoorian S, Lewis RA, et al. One year of netarsudil and latanoprost fixed-dose combination for elevated intraocular pressure: phase 3, randomized MERCURY-1 study. Ophthalmol Glaucoma. 2020;3(5):327-338.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
- Epithelial corneal edema, described as honeycomb or bullous, has been reported in some patients with pre-existing corneal stromal edema or following ocular procedures that could affect corneal endothelial function. Epithelial corneal edema typically resolves upon discontinuation of ROCKLATAN®. Advise patients to notify their physician if they experience eye pain or decreased vision while using ROCKLATAN®.
INDICATIONS AND USAGE
ROCKLATAN® (netarsudil and latanoprost ophthalmic solution) 0.02%/0.005%, is a fixed dose combination of a Rho kinase inhibitor and a prostaglandin F2α analogue indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
- Increased pigmentation of the iris, periorbital tissue (eyelid), and eyelashes can occur. Iris pigmentation likely to be permanent.
- Gradual change to eyelashes may include increased length, thickness, number, and misdirected growth of lashes. Usually reversible upon discontinuation of treatment.
- Use with caution in patients with a history of intraocular inflammation (iritis/uveitis). Should generally not be used in patients with active intraocular inflammation.
- Macular edema, including cystoid macular edema, has been reported with latanoprost. Use with caution in aphakic patients, pseudophakic patients with a torn posterior lens capsule, or patients with known risk factors for macular edema.
- Use with caution in patients with a history of herpetic keratitis. Avoid use in cases of active herpes simplex keratitis.
- There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface.
- Contact lenses should be removed prior to the administration of ROCKLATAN® and may be reinserted 15 minutes after administration.
INDICATIONS AND USAGE
ROCKLATAN® (netarsudil and latanoprost ophthalmic solution) 0.02%/0.005% is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
Adverse Reactions
The most common ocular adverse reaction observed in controlled clinical studies with ROCKLATAN® was conjunctival hyperemia which was reported in 59% of patients. Five percent of patients discontinued therapy due to conjunctival hyperemia. Other common ocular adverse reactions reported were: instillation site pain (20%), corneal verticillata (15%), and conjunctival hemorrhage (11%). Eye pruritus, visual acuity reduced, increased lacrimation, instillation site discomfort, and blurred vision were reported in 5-8% of patients.
Please click here for the ROCKLATAN® Full Prescribing Information.
