Powerful treatment.
Purposeful support.
Alcon offers support to help you get the most out of your ROCKLATAN® treatment.
INDICATION
- What is ROCKLATAN®?
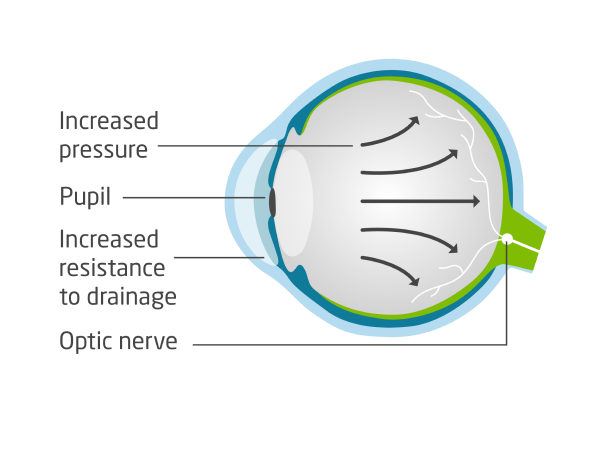
ROCKLATAN® (netarsudil and latanoprost ophthalmic solution) 0.02%/0.005% is a prescription eye drop that contains two medicines to reduce high eye pressure (intraocular pressure, or IOP) in patients with open-angle glaucoma or ocular hypertension.
IMPORTANT SAFETY INFORMATION
- ROCKLATAN® contains latanoprost, which may cause darkening of the eye color, darkening of the eyelid and eyelashes, and increased growth and thickness of eyelashes. Color changes may increase as long as ROCKLATAN® is administered, and eye color changes are likely to be permanent. Eyelash and eyelid changes may be reversible after discontinuation of ROCKLATAN®.
- Avoid allowing the tip of the bottle to contact the eye, surrounding structures, fingers, or any other surface in order to minimize contamination of the solution. Serious damage to the eye and subsequent loss of vision may result from using contaminated solution.
- If you develop another ocular condition (e.g., trauma or infection, or decreased vision with or without eye pain), have ocular surgery, or develop any ocular side effects, particularly conjunctivitis and eyelid reactions, you should immediately seek your healthcare provider’s advice about continuing to use ROCKLATAN®.
- The preservative in ROCKLATAN®, benzalkonium chloride, may be absorbed by soft contact lenses. Contact lenses should be removed prior to instillation of ROCKLATAN®, but may be reinserted 15 minutes after instillation.
- If you are using more than one eyedrop, the drugs should be administered at least 5 minutes apart.
- The most common side effect of ROCKLATAN® in clinical trials was red eyes (59% of patients). Five percent of patients stopped taking ROCKLATAN® due to red eyes. Other common side effects were: instillation site pain (20%), small deposits on the outer surface of the eye (15%), and broken blood vessels on the white of the eye (11%).
For additional information about ROCKLATAN®, talk to your healthcare provider and click here to see the full Prescribing Information.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
©2025 Alcon Inc. 11/25 US-ROC-25000064